Year 12 Chemistry begins with the concepts of open systems and closed systems. This will set us up to better understand static and dynamic equilibrium as well as non equilibrium…

What is the Mole Concept?
The mole concept is a way to measure the amount of a substance on a macroscopic scale. It allows chemists to bridge the gap between the atomic and macroscopic worlds by providing a common unit for counting entities like atoms, molecules, ions, and other particles.
In Year 11 Chemistry students are introduced to Quantitative Chemistry. This builds a foundation of knowledge from year 10 science such as:
- Understanding Atoms, Molecules & Compounds
- Writing Chemical Equations
- Different Types of Chemical Reactions
With this knowledge as our foundation, let’s explore the mole concept in more detail.
Atomic Mass
The atomic mass of individual atoms are very, very small. You may remember that together, the number of protons and the number of neutrons determine an element’s mass number:
mass number = protons + neutrons
For example, a single carbon atom with 6 protons, and 6 neutrons has a mass number of 12.
A property closely related to an atom’s mass number is its atomic mass. The atomic mass of a single atom is its total mass and is typically expressed in atomic mass units or amu.
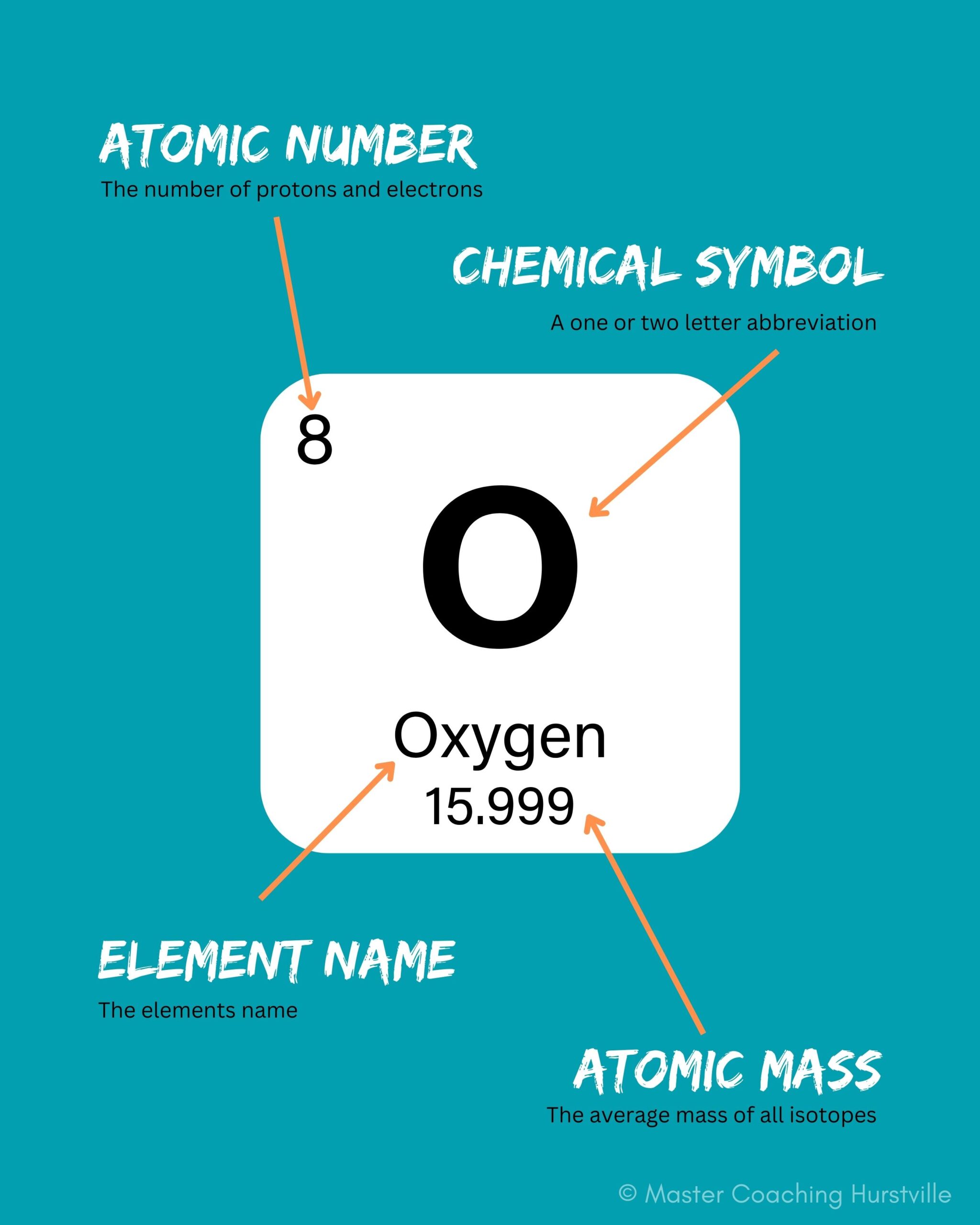
By definition, an atom of carbon with 6 neutrons, carbon-12, has an atomic mass of 12 amu. Other atoms don’t generally have round-number atomic masses for reasons that are a little beyond the scope of this blog post. In general, though, an atom’s atomic mass will be very close to its mass number, but will have some deviation in the decimal places. For example, oxygen has an atomic mass of 15.99 amu.
We can not see, touch or do chemistry with relative atomic mass because they are very small. To solve this issue the mole concept was created.
What is a Mole?
A mole is a unit that represents a specific number of entities, much like how a dozen represents 12 items. If I asked you to give me a dozen eggs, or a dozen pencils you would know how many to give me. A mole is the same concept on a much larger scale. A dozen is 12 entities, and a mole is 602,200,000,000,000,000,000,000 entities. This number is also called Avogadro’s number and can be more succinctly expressed as 6.022 × 10²³.
In order to do chemistry with quantities of atoms and molecules in a practical and meaningful way we need to work with them at a larger scale than individual atoms. If we work with; for example; 1 mole of carbon, we are now working with a scale we can touch and see.
But at how do we measure out 1 mole of carbon, or 1 mole of oxygen? To know that we need to know an elements molar mass.
What is Molar Mass?
Molar mass is the mass in grams of 1 mole of that substance.
An elements molar mass is numerically equal to its atomic mass. For example 1 mole of carbon (which has an atomic mass of 12 amu) has a molar mass of 12 g/mol. Or, 1 mole of oxygen (which has an atomic mass of 15.99 amu) has a molar mass of 15.99 g/mol.
If you are confused it may be helpful to remember that we can often measure items in multiple ways. For example, 1 dozen eggs is equal to 12 eggs AND approximately 600g. Similarly 1 mole of carbon is equal to 6.022 × 10²³ atoms of carbon AND 12 g/mol.
Conversions with the Mole Concept
The mole concept allows chemists to convert between the mass of a substance and the number of moles it contains using its molar mass. This is done using the formula:
moles = mass (g) / molar mass (g/mol).
So if we have 4 moles of carbon, and it has a molar mass of 12g/mol we can figure out how many grams that 4 moles would weigh.
4 = x / 12
x = 12 x 4
x = 48 grams
Or if we know we have 60 grams of carbon we can figure out how many moles that’s is:
y = 60/12
y = 5 moles
There are 5 moles of carbon in 60 grams. That is 5 x 6.022 × 10²³ atoms in those 60 grams.
In summary, the mole concept provides a quantitative foundation for understanding the amounts of substances involved in chemical reactions and allows chemists to work with quantities of atoms and molecules in a practical and meaningful way.
Need Help with Year 11 Chemistry?

HSC Chemistry Tutor
Master Coaching offers one on one tutoring for HSC Chemistry. We are located in Hurstville, Sydney, and also offer online tutoring to students across NSW.



