Year 12 Chemistry begins with the concepts of open systems and closed systems. This will set us up to better understand static and dynamic equilibrium as well as non equilibrium…

Chemical reactions happen all around us. When a chemical reaction occurs the atoms are rearranged to form new substances.
Year 10 science provides some foundational chemistry knowledge to help students develop a deeper understanding of the world around them. For students who want to excel in their studies, or perhaps take Chemistry as an HSC subject, making sure that you understand the foundational knowledge presented in year 10 science is crucial.
Previously on the blog we looked at the difference between atoms, molecules and compounds, and writing chemical equations. Today on the blog we will discussing different types of chemical reactions .

Combustion
Cooking with gas, a bush fire and burning petrol in a car are examples of combustion. It involves combining a substance with oxygen to produce heat and light. For example, Magnesium (Mg) burns in oxygen (O2) to give magnesium oxide (MgO).
2 Mg (s) + O2 (g) → 2 MgO (g)
Acid/base reactions
Acid/base reactions involve water and occur everywhere that water is found. Taking bicarbonate for an upset stomach, digesting food, cleaning copper with acid and adjusting the pH in garden soil all involve acid/base reactions.

Corrosion
Cars rusting, aluminium window frames becoming crusty, copper and silver going black, are all forms of corrosion. The most common form of corrosion involves the combination of metal with oxygen to form a metal oxide. Rust is iron oxide while the white powder on aluminium is aluminium oxide.
Precipitation
We often think of rain when we hear the word precipitation, but in chemistry it can also refer to solids being formed in a liquid solution.
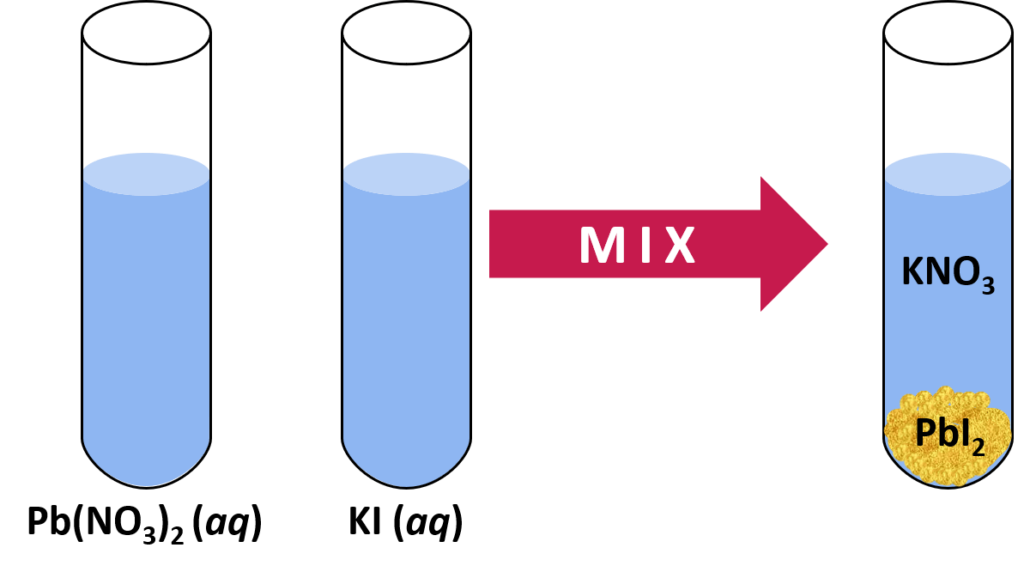
When writing a chemical reaction, the presence of a precipitate is indicated by following the chemical formula with an (s) representing the precipitate (insoluble salt).
For example is when lead nitrate reacts with potassium iodide a yellow precipitate of lead iodide is formed.
Pb(NO3)2(aq) + 2KI (aq) → PbI2(s) + 2KNO3(aq)
Neutralisation
The neutralisation reaction involves the hydrogen ion and the hydroxide ion reacting together to form water. The acid ionises to produce the hydrogen ion (H+) and the base dissociates to form the hydroxide ion (OH–). The nuetralistion reaction is:
H+(aq) + OH– (aq) → H20(lq)
The above is referred to as the net ionic reaction.
Decomposition
A decomposition reaction is a chemical reaction in which one reactant breaks down into two or more products. One major application of decomposition reactions is in the extraction of metals from their ores.
For example, zinc can be obtained from carbonate ore (calamine) by subjecting it to a decomposition reaction at high temperatures. This involves a two step process. The first reaction is the decomposition of zinc carbonate to form zinc oxide and carbon dioxide.
ZnCO3(s) → ZnO(s) + CO2(g)
The second step is a purification which involves using zinc oxide and carbon monoxide to extract the zinc from the zinc oxide to form pure zinc.
ZnO(s) + CO(g) → Zn(s) + CO2(g)
Getting Help with Chemical Reactions
There is a wide range of skills and knowledge covered in year 7-10 science. It is no wonder that many students find it challenging. Having a full comprehension of what is being taught in these junior years will set you up for success in the HSC.

Year 7 – 10 Science Tutoring
Master Coaching offers one on one tutoring for year 7-10 science. We are located in Hurstville Sydney, and also offer online tutoring to students across NSW.

Head Start to Year 11 Chemistry
Master Coaching is offering a holiday program to students who have finished year 10, and are about to start year 11 Chemistry. The best way to succeed in the HSC is to make sure students understand the foundations so they can follow along with what is being taught to them when year 11 gets underway.



