Year 12 Chemistry begins with the concepts of open systems and closed systems. This will set us up to better understand static and dynamic equilibrium as well as non equilibrium…

Year 10 science provides some foundational chemistry knowledge to help students better understand the world around them. This includes understanding the difference between atoms, molecules, and compounds.
For students who want to excel in their studies, or perhaps take Chemistry as an HSC subject, making sure that you understand the foundational knowledge presented in year 10 science is crucial. This basic information will set you up to understand chemical reactions, and how to balance them.
What is an Atom?
Atoms are the smallest building blocks of matter and make up everything around us. Every atom has a center called a nucleus, which is made of particles called protons and neutrons. Electrons move in electron shells around the nucleus.
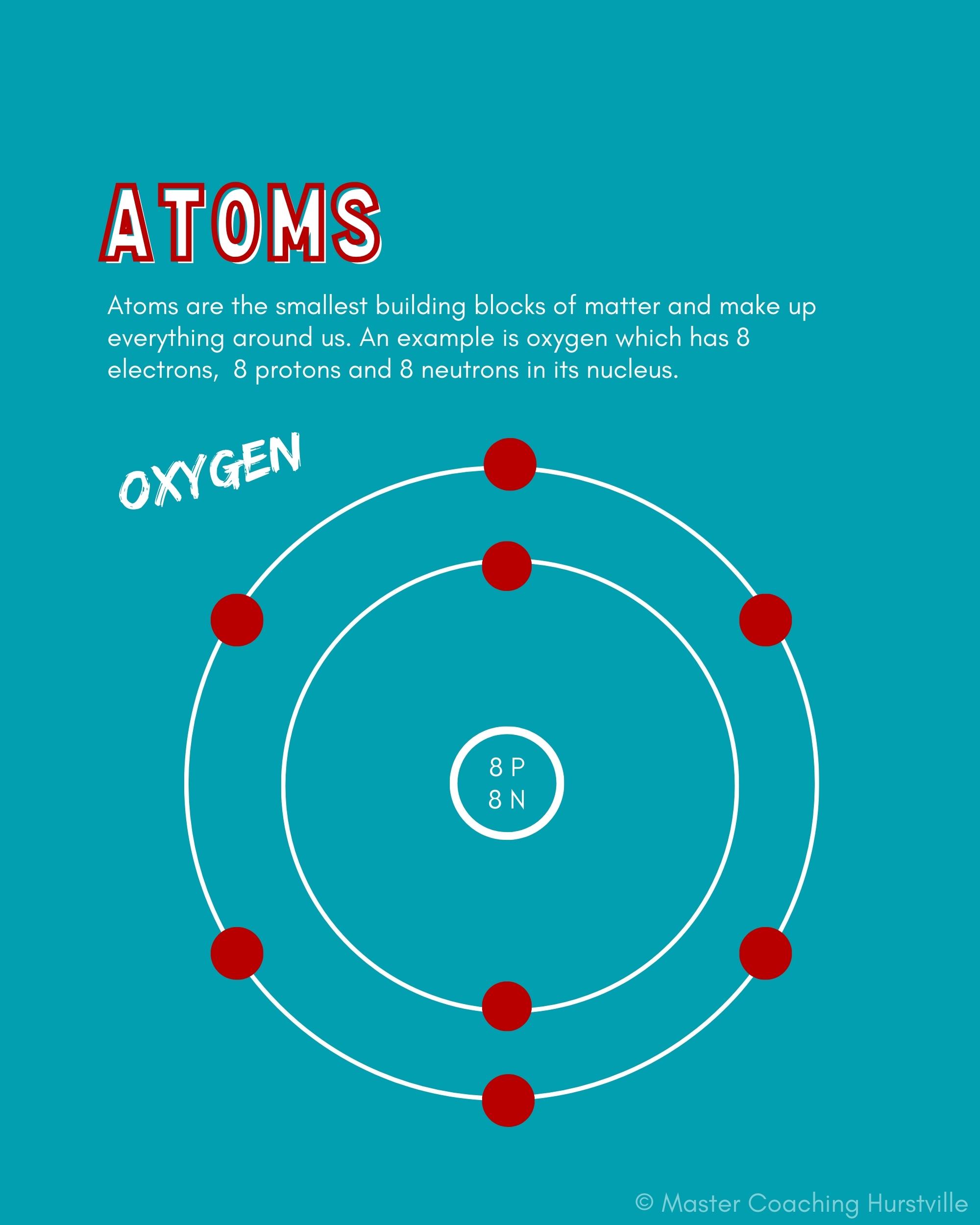
Recap of some basic facts about atoms:
You most likely were introduced to atoms in year 9 science and have learnt many interesting things about them. Before moving on to molecules and compounds let’s make sure we understand some basic facts about atoms that we will build upon:
- all matter is made of atoms
- atoms have a mass
- atoms are composed of protons, neutrons and electrons
- the atom is the smallest unit of an element
- atoms can be represented by a symbol eg. H is the symbol for Hydrogen
- we can distinguish between atoms by comparing information about the numbers of protons, neutrons and electrons eg, Hydrogen has a single proton and a single electron while oxygen has 8 protons, 8 neutrons, and 8 electrons.
- the atomic structure and properties of elements are used to organise them in the Periodic Table
If those facts seemed simple to you; you are ready to move on and learn about molecules and compounds.
What is a Molecule?
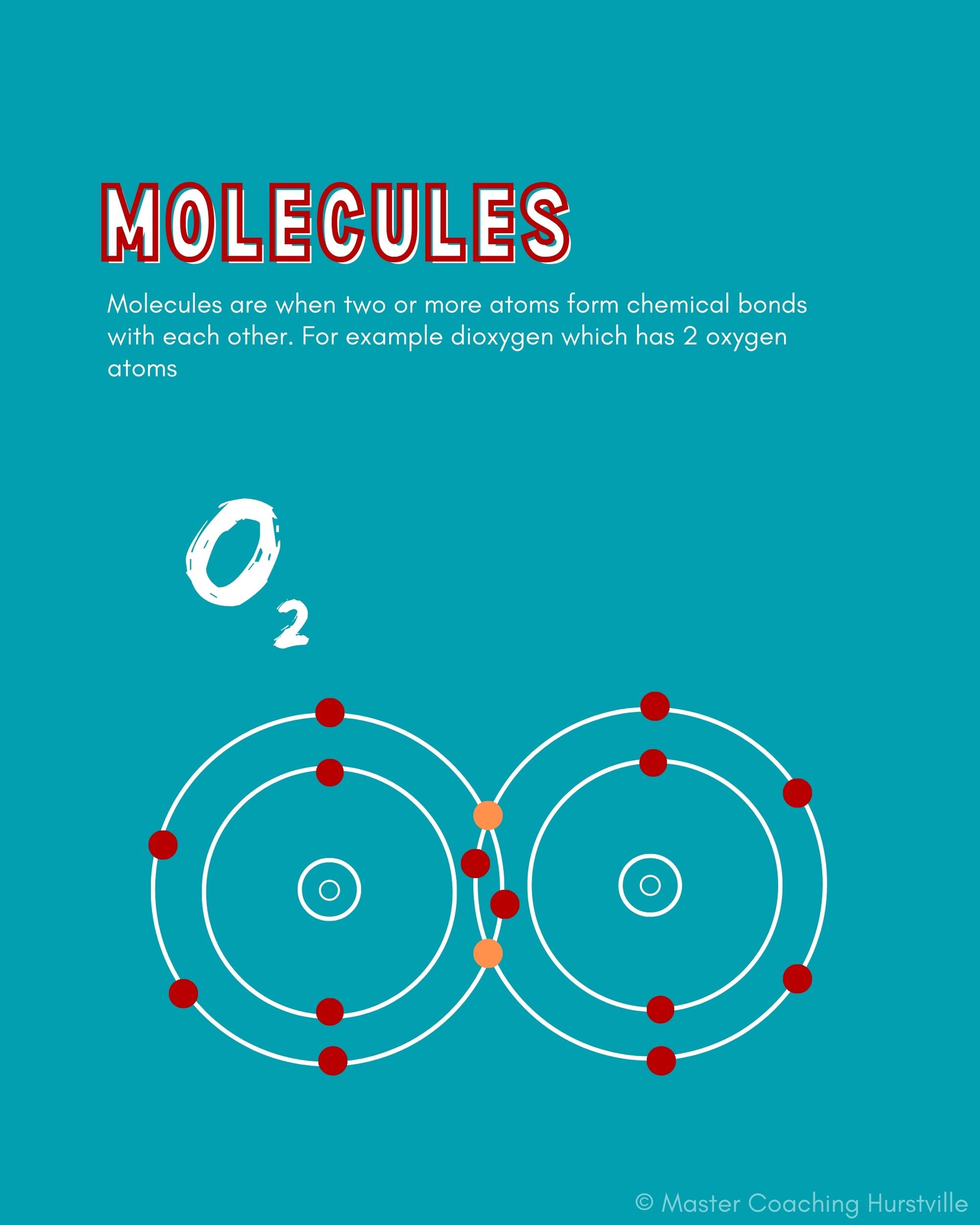
Molecules are when two or more atoms form chemical bonds with each other. It doesn’t matter if the atoms are the same element or are different from each other.
Here are some examples of common molecules:
- H2O (water)
- N2 (nitrogen)
- O3 (ozone)
- CaO (calcium oxide)
- C6H12O6 (glucose, a type of sugar)
- NaCl (table salt)
Single atoms of elements are not molecules. A single oxygen, O, is not a molecule. When oxygen bonds to itself (e.g., O2, O3) or to another element (e.g., carbon dioxide or CO2), molecules are formed.
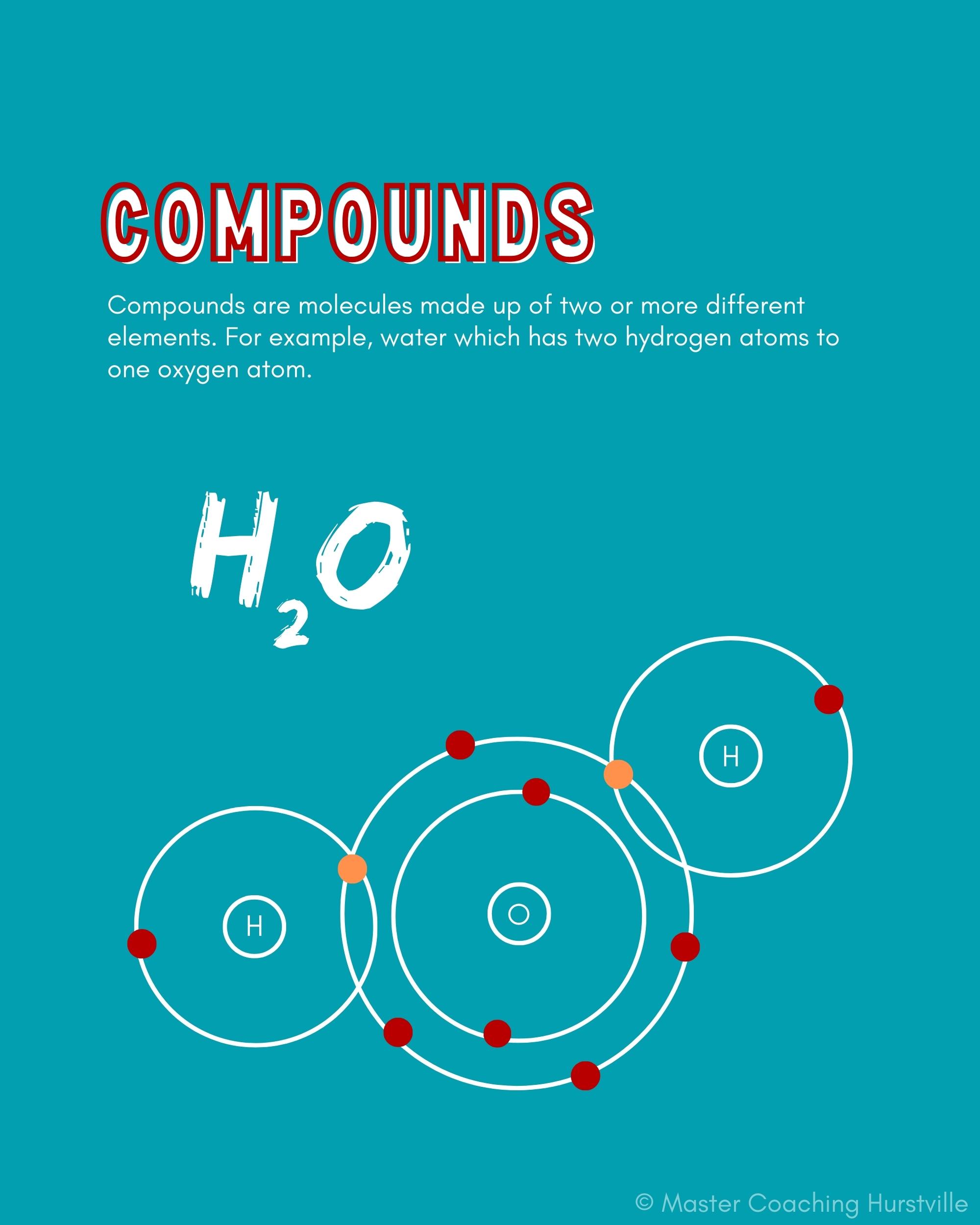
What is a Compound?
Compounds are molecules that are made up of two or more elements. H2O (water), CaO (calcium oxide), C6H12O6 (glucose) and NaCl (table salt) are compounds, while N2 (nitrogen) and O3 (ozone) are not compounds. All compounds are molecules; not all molecules are compounds.
Why Do We Need to Know this in Year 10 Science?
Understanding the difference between atoms, molecules and compounds will give you the foundational knowledge you need to understand chemical reactions, writing chemical equations, and balancing chemical equations.
Getting Help With Year 10 Science
There is a wide range of skills and knowledge covered in year 7-10 science. It is no wonder that many students find it challenging. Having a full comprehension of what is being taught in these junior years will set you up for success in the HSC.

Year 7 – 10 Science Tutoring
Master Coaching offers one on one tutoring for year 7-10 science. We are located in Hurstville Sydney, and also offer online tutoring to students across NSW.

Head Start to Year 11 Chemistry
Master Coaching is offering a holiday program to students who have finished year 10, and are about to start year 11 Chemistry. The best way to succeed in the HSC is to make sure students understand the foundations so they can follow along with what is being taught to them when year 11 gets underway.



